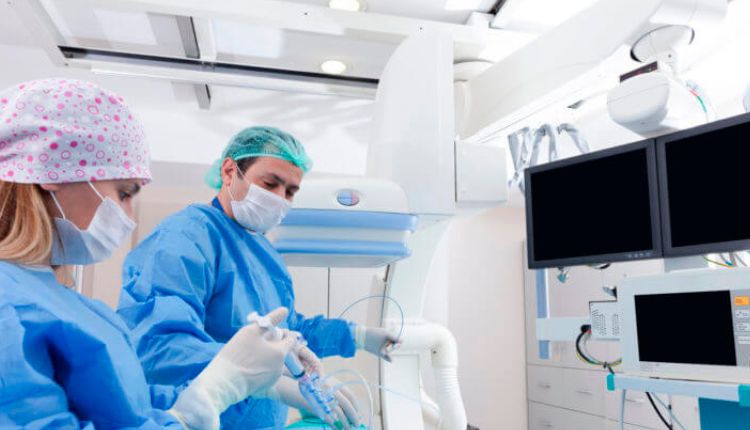
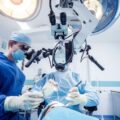
A clinical trial is a controlled study of a new drug or treatment. Participants are monitored to ensure their safety and wellbeing. This research helps develop better treatments for future use. Cell studies test treatments on cancer cells in a lab dish. If the results are promising, the researchers move to animal trials.
Benefits
Many people choose to participate in clinical research because they want the chance to try new treatments for their health condition before they are available for everyone. They also enjoy the opportunity to feel like they are playing a more active role in their health care. In addition, some participants have access to specialized medical care that is not otherwise available. However, it is important to remember that all clinical trials have risks. Before starting a clinical trial, you should talk to the study’s researchers and get all of your questions answered. This process is called informed consent. It is a legal requirement before participating in any clinical trial.
Most clinical studies are monitored by an Institutional Review Board (IRB), which is a group of doctors, statisticians and community members that ensures that the trials are ethical and that the participants’ rights are protected. In addition, most IRBs follow a set of international guidelines known as Good Clinical Practice.
Eligibility
Clinical trials in usa are research studies that evaluate new tests and treatments. The results of these studies can help doctors and patients decide if the treatment is safe and effective. These studies may involve drugs, cells and other biological products, surgical procedures, radiological procedures, devices, behavioural treatments or preventive care. All clinical trials must be carefully designed and reviewed before they can start.
Choosing to participate in a clinical trial is a personal decision. You should carefully consider your interests, goals and options, and discuss them with your health care provider or other trusted members of your family. You should also get information about your medical history and current medications before participating in a clinical trial. You should also ask about the potential benefits and risks of participating in a clinical trial. It is important to remember that participating in a clinical trial does not guarantee that you will receive any medication or other treatment. Trials may be stopped if they are unsafe for participants or if the medication does not improve health outcomes.
Procedures
The procedures associated with a clinical trial vary from one trial to the next, but all trials must follow federal and international patient privacy laws and good clinical practice. They must also adhere to the ethical principle of primum non nocere (first, do no harm). Before a trial begins, research staff explain the study and its benefits and risks to the potential participants. They then screen them to make sure they are eligible for the trial. Once the trial starts, they must continue to screen and monitor participants’ health throughout the study. This includes tests to ensure the trial is working as intended.
During phase 3, researchers increase the number of participants to several thousand and continue testing for safety, effectiveness and side effects. This large scale of randomized and blind testing allows researchers to get the most accurate data possible before pharmaceutical companies submit applications to the FDA to take the drug to market.
Costs
Taking part in a clinical trial has both benefits and costs. Participants may receive new treatments that can improve their health and quality of life, but they also have to pay for extra doctor visits, tests, and travel expenses. The amount of money paid varies from study to study, and the informed consent form will describe what is covered. Most trials will cover the cost of the drug or treatment, but some will not. Some studies will also reimburse participants for co-pays and deductibles.
While the exact price of a clinical trial will depend on the type of study, it is possible to estimate costs in advance. Many CROs have analysts or project managers who can help with budgeting, and there are also independent pharmacy consultants and pharma attorneys who can assist. The table below describes the general cost sections included in a typical CRO quotation. These figures are not a complete list of costs, but they are helpful in estimating overall project costs.
More Words
Clinical trials test new medicines, medical devices, or treatments to see if they work and are safe. They are conducted by universities, hospitals, and private groups. Participants in a trial are randomly assigned to a treatment or control group. The people in the treatment group get the medicine or procedure being tested.